Alban ARSENE
C4PMD CEO and Founder
30 years of background in operations, processes, products, facilities and supply chain within pharmaceutical industry; aseptic, sterile process and medical devices class III.
Strong knowledges of FDA rules regarding cGMP's (21CFR 210,211,820) & MDSAP audit program. Global engineering and start-up of factory buildings.



Project#1 : equipment implementation
How C4PMD handle technical projects?
Industrial master plan based on the 4 pillars
- by Design : Product process expectations
- by Quality : Product quality expectations
- by Regulatory : Product regulatory expectations
- by Manufacturing : Product manufacturing expectations
Project PMO
- project management
- Costs and schedule
- Project implementation
Equipment implementation
- Process risk analyses
- Design review of the equipment
- Factory Acceptance Test (FAT): Test capitalisation
- Installation Qualification
- Operational Qualification
- Process validation
Release to production
Steam sterilizator

Hyaluronic Acid filling station

Project#2 : Iso 17665-1 remediation
How to sterilize thermolabile product as hyaluronic acid pre-filled syringes?
Sterilization for a thermolabile product is very challenging.
For assuming the day to day the Sterility Assurance Level of a medical device or a parenteral drug, legal manufacturer should follow the ISO 17665-1 norm
C4PMD is your expert, for the sterilization validation up to the release to production.
C4PMD will support te legal manufacturer.
Validation strategy
- Validation master plan
- Qualification of the sterilizer
- Process validation for the sterilisation itself
- Sterilization validation report with the SAL
- Validation summary report
C4PMD offer a consistant training about the sterilization validation in accordance with the ISO 17665-1:
- Sterilization basic concept
- Sterilization detail concept
- Validation strategy
- D value & Z value
- Bioligical Indicators
- SAL calculations
Steam sterilizator

Sterilization training

Project#3 : DHF remediation
Manufacturing process validation
Design transfert remediation in accordance with MDR 2017/45
Validation prospective in accordance with a retrospective manufacturing data available
C4PMD is your expert, for the manufacturing process validation
C4PMD will support te legal manufacturer.
Validation strategy
- Validation master plan
- Process risk analyses
- Worst case challenge definition as output of the process risk analyse
- OQ process protocol with worst cases challenges
- PQ process portocol as routine manufacturing batches
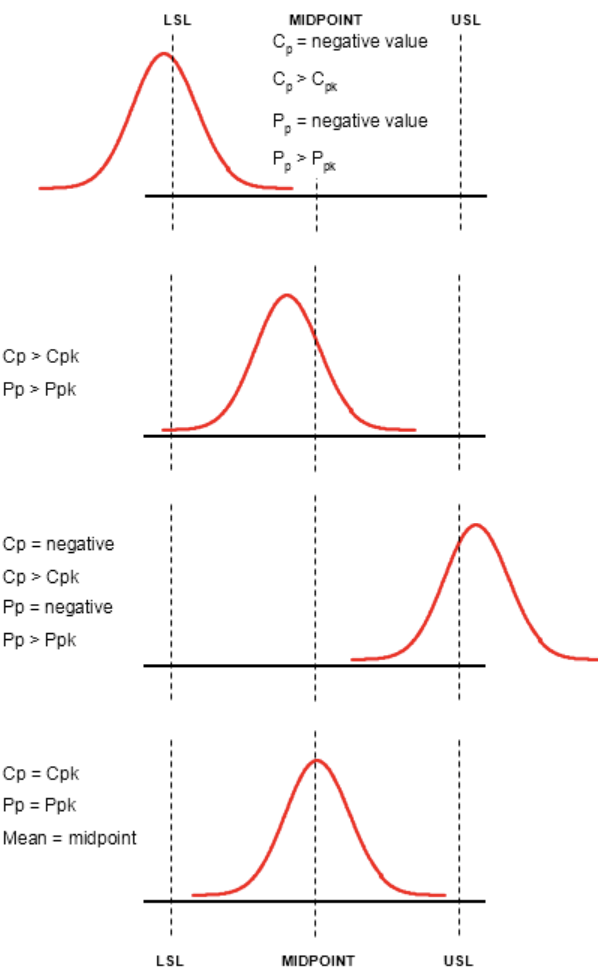
- Cpk and Ppk evaluation thanks to Minitab software
- Validation summary report
Cpk & Ppk manufacturing process validation
C4PMD : your expert in life sciences
Copyright ©. Tous droits réservés.
